Roots Analysis has announced the addition of “Pharmaceutical Contract Manufacturing Market (3rd Edition), 2021-2030” report to its list of offerings.
To order this 510 page report, which features 180+ figures and 175+ tables, please visit
Key Market Insights
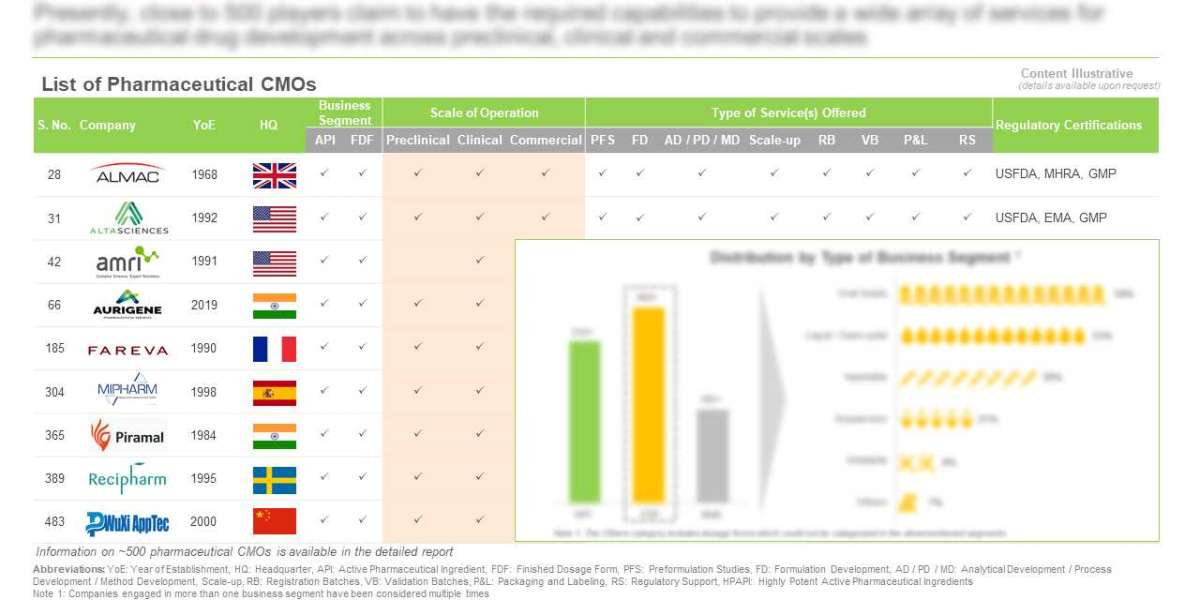
- Presently, close to 500 players claim to have the required capabilities to provide a wide array of services for pharmaceutical drug development across preclinical, clinical and commercial scales.
- The market is characterized by the presence of both established players and new entrants that offer end-to-end services, ranging from pre-formulation studies to regulatory support.
- With more than 1,350 facilities, pharmaceutical contract manufacturers have established global presence; majority of these players are based in emerging geographies.
- In order to meet the rising demand for APIs and finished products, CMOs have made elaborate investments to expand their existing capacities and capabilities; this trend is most pronounced in the US and India.
- In pursuit of a competitive edge and to eventually establish themselves as one-stop shops, stakeholders are actively consolidating their capabilities related to small molecule manufacturing through mergers and acquisitions.
- The installed global contract manufacturing capacity, spread across various geographies, is presently estimated to be close to 160 million litres.
- Though existing capacity is sufficient to meet the current annual demand of small molecule APIs, we anticipate that stakeholders will continue to invest in building incremental capacity to meet the long-term demand.
- Increase in number of approvals and manufacturing complexity related to small molecule drugs is anticipated to drive the growth in contract manufacturing market at a CAGR of 6.5%, till 2030.
- The projected future opportunity is likely to be well-distributed, in terms of service revenues, across different types of packaging forms, end-users and key geographical regions.
For more information, please visit https://www.rootsanalysis.com/reports/view_document/pharmaceutical-contract-manufacturing-market/191.html or email sales@rootsanalysis.com
Table of Contents
PREFACE
1.1. Scope of the Report
1.2. Research Methodology
1.3. Key Questions Answered
1.4. Chapter Outlines
- EXECUTIVE SUMMARY
- INTRODUCTION
3.1. Chapter Overview
3.2. Types of Manufacturers
3.3. Overview of Pharmaceutical Contract Manufacturing
3.4. Evolution of Pharmaceutical Contract Manufacturing
3.4.1. Traditional Pharmaceutical CMOs
3.4.2. Modern Pharmaceutical CMOs
3.5. Need for Outsourcing in the Pharmaceutical Industry
3.6. Recent Trends in the Pharmaceutical Contract Manufacturing Industry
3.6.1. Tactical Partnerships
3.6.2. Integrated End-to-End Business Model
3.6.3. Software Service Providers
3.7. Services Offered by CMOs
3.8. Key Considerations While Selecting a CMO Partner
3.9. Risks and Challenges Associated with Outsourcing Pharmaceutical Manufacturing Operations
- REGULATORY LANDSCAPE FOR PHARMACEUTICAL CONTRACT MANUFACTURERS
4.1. Chapter Overview
4.2. Regulatory Guidelines in North America
4.2.1. The US Scenario
4.2.2. Canadian Scenario
4.3. Regulatory Guidelines in Europe
4.4. Regulatory Guidelines in Asia-Pacific and Rest of the World
4.4.1. Chinese Scenario
4.4.2. Indian Scenario
4.4.3. Japanese Scenario
4.4.4. South Korean Scenario
4.4.5 Australian Scenario
4.4.6. Brazilian Scenario
4.5. Analysis of Pharmaceutical CMOs by Approval Received from Regulatory Authorities
4.6. Regional Summary of Regulatory Scenario (Bubble Analysis)
- PHARMACEUTICAL CMOs: CURRENT MARKET LANDSCAPE
5.1. Chapter Overview
5.2. Pharmaceutical CMOs: List of Industry Players
5.2.1. Analysis by Year of Establishment
5.2.2. Analysis by Company Size
5.2.3. Analysis by Location of Headquarters
5.2.4. Analysis by Type of Business Segment
5.2.5. Analysis by Type of API Manufactured
5.2.6. Analysis by Type of FDF Offered
5.2.7. Analysis by Type of Molecule
5.2.8. Analysis by Type of Service(s) Offered
5.2.9. Analysis by Type of Primary Packaging Form Offered
5.2.10. Analysis by Scale of Operation
5.2.11. Analysis by Location of Manufacturing Facility
5.2.11.1 Pharmaceutical Contract Manufacturing Facilities in North America
5.2.11.2 Pharmaceutical Contract Manufacturing Facilities in Europe
5.2.11.3 Pharmaceutical Contract Manufacturing Facilities in Asia-Pacific and Rest of the World
- REGIONAL CAPABILITY
6.1. Chapter Overview
6.2. Key Assumptions and Methodology
6.3. Regional Capability Analysis of Pharmaceutical CMOs
6.3.1 Analysis of CMOs based in North America
6.3.2. Analysis of CMOs based in Europe
6.3.3. Analysis of CMOs based in Asia-Pacific and Rest of the World
- COMPANY PROFILES
7.1. Chapter Overview
7.2. Key Benefits Key Benefits Offered by the One-Stop-Shop Model
7.3 Profiles of Players Operating as One-Stop-Shops
7.4. North America
7.4.1. AMRI Global
7.4.1.1. Company Overview
7.4.1.2. Service Portfolio
7.4.1.3. Manufacturing Capabilities and Facilities
7.4.1.4. Recent Developments and Future Outlook
7.4.2. Altasciences
7.4.2.1. Company Overview
7.4.2.2. Service Portfolio
7.4.2.3. Manufacturing Capabilities and Facilities
7.4.2.4. Recent Developments and Future Outlook
7.4.3. Cambrex
7.4.3.1. Company Overview
7.4.3.2. Service Portfolio
7.4.3.3. Manufacturing Capabilities and Facilities
7.4.3.4. Recent Developments and Future Outlook
7.4.4. Catalent
7.4.4.1. Company Overview
7.4.4.2. Financial Information
7.4.4.3. Service Portfolio
7.4.4.4. Manufacturing Capabilities and Facilities
7.4.4.5. Recent Developments and Future Outlook
7.4.5. DPT Laboratories
7.4.5.1. Company Overview
7.4.5.2. Service Portfolio
7.4.5.3. Manufacturing Capabilities and Facilities
7.4.5.4. Recent Developments and Future Outlook
7.4.6. Thermo Fisher Scientific
7.4.6.1. Company Overview
7.4.6.2. Financial Information
7.4.6.3. Service Portfolio
7.4.6.4. Manufacturing Capabilities and Facilities
7.4.6.5. Recent Developments and Future Outlook
7.5. Europe
7.5.1. Aenova Group
7.5.1.1. Company Overview
7.5.1.2. Financial Information
7.5.1.3. Service Portfolio
7.5.1.4. Manufacturing Capabilities and Facilities
7.5.1.5. Recent Developments and Future Outlook
7.5.2. Almac
7.5.2.1. Company Overview
7.5.2.2. Service Portfolio
7.5.2.3. Recent Developments and Future Outlook
7.5.3. Corden Pharma
7.5.3.1. Company Overview
7.5.3.2. Service Portfolio
7.5.3.3. Manufacturing Capabilities and Facilities
7.5.3.4. Recent Developments and Future Outlook
7.5.4. Fresenius Kabi
7.5.4.1. Company Overview
7.5.4.2. Financial Information
7.5.4.3. Service Portfolio
7.5.4.4. Manufacturing Capabilities and Facilities
7.5.4.5. Recent Developments and Future Outlook
7.5.5. Glatt
7.5.5.1. Company Overview
7.5.5.2. Service Portfolio
7.5.5.3. Manufacturing Capabilities and Facilities
7.5.5.4. Recent Developments and Future Outlook
7.5.6. Hovione
7.5.6.1. Company overview
7.5.6.2. Service Portfolio
7.5.6.3. Manufacturing Capabilities and Facilities
7.5.6.4. Recent Developments and Future Outlook
7.5.7. Recipharm
7.5.7.1. Company Overview
7.5.7.2. Financial Information
7.5.7.3. Service Portfolio
7.5.7.4. Manufacturing Capabilities and Facilities
7.5.7.5. Recent Developments and Future Outlook
7.5.8. Siegfried
7.5.8.1. Company Overview
7.5.8.2. Financial Information
7.5.8.3. Service Portfolio
7.5.8.4. Manufacturing Capabilities and Facilities
7.5.8.5. Recent Developments and Future Outlook
7.6. Asia-Pacific and Rest of the World
7.6.1. CMIC Group
7.6.1.1. Company Overview
7.6.1.2. Financial Information
7.6.1.3. Service Portfolio
7.6.1.4. Recent Developments and Future Outlook
7.6.2. Nectar Lifesciences
7.6.2.1. Company Overview
7.6.2.2. Financial Information
7.6.2.3. Service Portfolio
7.6.2.4. Manufacturing Capabilities and Facilities
7.6.2.5. Recent Developments and Future Outlook
7.6.3. Syngene
7.6.3.1. Company Overview
7.6.3.2. Financial Information
7.6.3.3. Service Portfolio
7.6.3.4. Manufacturing Capabilities and Facilities
7.6.3.5. Recent Developments and Future Outlook
7.6.4. WuXi AppTec
7.6.4.1. Company Overview
7.6.4.2. Financial Information
7.6.4.3. Service Portfolio
7.6.4.4. Manufacturing Capabilities and Facilities
7.6.4.5. Recent Developments and Future Outlook
- MAKE VERSUS BUY DECISION FRAMEWORK
8.1. Chapter Overview
8.2. Assumptions and Key Parameters
8.3. Pharmaceutical Contract Manufacturers: Make versus Buy Decision Making
8.3.1. Scenario 1
8.3.2. Scenario 2
8.3.3. Scenario 3
8.3.4. Scenario 4
8.4. Concluding Remarks
- MERGERS AND ACQUISITIONS
9.1. Chapter Overview
9.2. Merger and Acquisition Models
9.3. Pharmaceutical Contract Manufacturing: Mergers and Acquisitions
9.3.1. Cumulative Year-wise Trend
9.3.2. Analysis by Type of Agreement
9.3.3. Analysis by Type of Acquisition
9.3.4. Analysis by Focus Area
9.3.5. Analysis by Type of Business Segment
9.3.6. Analysis by Type of Service(s) Offered
9.3.7. Analysis by Scale of Operation
9.3.8. Analysis by Geography
9.3.9. Analysis by Location of Manufacturing Facility
9.3.10. Most Active Acquirers: Analysis by Number of Acquisitions
9.3.11. Analysis by Key Value Drivers
9.3.11.1. Analysis by Year of Acquisition and Key Value Drivers
9.4. Key Acquisitions: Analysis by Deal Multiples
- RECENT EXPANSIONS
10.1. Chapter Overview
10.2. Pharmaceutical Contract Manufacturing Market: Expansions
10.2.1. Cumulative Year-wise Distribution
10.2.2. Analysis by Purpose of Expansion
10.2.3. Analysis by Year and Purpose of Expansion
10.2.4. Analysis by Type of Business Segment
10.2.5. Analysis by Type of Molecule
10.2.6. Analysis by Type of Service(s) Offered
10.2.7. Analysis by Scale of Operation
10.2.8. Analysis by Capital Invested
10.2.9. Analysis by Location of Headquarters
10.2.10. Analysis by Purpose of Expansion and Location of Headquarters
10.2.11. Analysis by Location of Manufacturing Facility
10.2.12. Most Active Players: Analysis by Number of Recent Expansions
- CAPACITY ANALYSIS
11.1. Chapter Overview
11.2. Key Assumptions and Methodology
11.3. Pharmaceutical Contract Manufacturing: Global Production Capacity
11.3.1. Analysis by Company Size
11.3.2. Analysis by Scale of Operation
11.3.3. Analysis by Location of Headquarters
11.3.3.1. Analysis of Capacity Installed in North America
11.3.3.2. Analysis of Capacity Installed in Europe
11.3.3.3. Analysis of Capacity Installed in Asia-Pacific
11.4. Concluding Remarks
- DEMAND ANALYSIS
12.1. Chapter Overview
12.2. Key Assumptions and Methodology
12.3. Overall Annual Demand for Small Molecule APIs, 2021-2030
12.3.1. Analysis by Scale of Operation
12.4. Correlation Between Annual Demand and Capacity
- MARKET FORECAST
13.1. Chapter Overview
13.2. Forecast Methodology and Key Assumptions
13.3. Global Pharmaceutical Contract Manufacturing Market, 2021-2030
13.3.1. Pharmaceutical Contract Manufacturing Market, 2021 and 2030: Distribution by Business Segment
13.1.2. Pharmaceutical Contract Manufacturing Market, 2021 and 2030: Distribution by Type of API Manufactured
13.1.3. Pharmaceutical Contract Manufacturing Market, 2021 and 2030: Distribution by Type of FDF Offered
13.1.4. Pharmaceutical Contract Manufacturing Market, 2021 and 2030: Distribution by Scale of Operation
13.1.5. Pharmaceutical Contract Manufacturing Market, 2021 and 2030: Distribution by Type of Packaging Form
13.1.6. Pharmaceutical Contract Manufacturing Market, 2021 and 2030: Distribution by Type of End-Users
13.1.7. Pharmaceutical Contract Manufacturing Market, 2021 and 2030: Distribution by Geography
13.1.7.1. Pharmaceutical Contract Manufacturing Market in North America, 2021-2030
13.1.7.1.1. Pharmaceutical Contract Manufacturing Market in North America, 2021-2030: Distribution by Business Segment
13.1.7.1.2. Pharmaceutical Contract Manufacturing Market in North America, 2021-2030: Distribution by Type of API Manufactured
13.1.7.1.3. Pharmaceutical Contract Manufacturing Market in North America, 2021-2030: Distribution by Type of FDF Offered
13.1.7.1.4. Pharmaceutical Contract Manufacturing Market in North America, 2021-2030: Distribution by Scale of Operation
13.1.7.1.5. Pharmaceutical Contract Manufacturing Market in North America, 2021-2030: Distribution by Type of Packaging Form
13.1.7.1.6. Pharmaceutical Contract Manufacturing Market in North America, 2021-2030: Distribution by Type of End-Users
13.1.7.2. Pharmaceutical Contract Manufacturing Market in Europe, 2021-2030
13.1.7.2.1. Pharmaceutical Contract Manufacturing Market in Europe, 2021-2030: Distribution by Business Segment
13.1.7.2.2. Pharmaceutical Contract Manufacturing Market in Europe, 2021-2030: Distribution by Type of API Manufactured
13.1.7.2.3. Pharmaceutical Contract Manufacturing Market in Europe, 2021-2030: Distribution by Type of FDF Offered
13.1.7.2.4. Pharmaceutical Contract Manufacturing Market in Europe, 2021-2030: Distribution by Scale of Operation
13.1.7.2.5. Pharmaceutical Contract Manufacturing Market in Europe, 2021-2030: Distribution by Type of Packaging Form
13.1.7.2.6. Pharmaceutical Contract Manufacturing Market in Europe, 2021-2030: Distribution by Type of End-Users
13.1.7.3. Pharmaceutical Contract Manufacturing Market in Asia-Pacific, 2021-2030
13.1.7.3.1. Pharmaceutical Contract Manufacturing Market in Asia-Pacific, 2021-2030: Distribution by Business Segment
13.1.7.3.2. Pharmaceutical Contract Manufacturing Market in Asia-Pacific, 2021-2030: Distribution by Type of API Manufactured
13.1.7.3.3. Pharmaceutical Contract Manufacturing Market in Asia-Pacific, 2021-2030: Distribution by Type of FDF Offered
13.1.7.3.4. Pharmaceutical Contract Manufacturing Market in Asia-Pacific, 2021-2030: Distribution by Scale of Operation
13.1.7.3.5. Pharmaceutical Contract Manufacturing Market in Asia-Pacific, 2021-2030: Distribution by Type of Packaging Form
13.1.7.3.6. Pharmaceutical Contract Manufacturing Market in Asia-Pacific, 2021-2030: Distribution by Type of End-Users
13.1.7.4. Pharmaceutical Contract Manufacturing Market in Latin America, 2021-2030
13.1.7.4.1. Pharmaceutical Contract Manufacturing Market in Latin America, 2021-2030: Distribution by Business Segment
13.1.7.4.2. Pharmaceutical Contract Manufacturing Market in Latin America, 2021-2030: Distribution by Type of API Manufactured
13.1.7.4.3. Pharmaceutical Contract Manufacturing Market in Latin America, 2021-2030: Distribution by Type of FDF Offered
13.1.7.4.4. Pharmaceutical Contract Manufacturing Market in Latin America, 2021-2030: Distribution by Scale of Operation
13.1.7.4.5. Pharmaceutical Contract Manufacturing Market in Latin America, 2021-2030: Distribution by Type of Packaging Form
13.1.7.4.6. Pharmaceutical Contract Manufacturing Market in Latin America, 2021-2030: Distribution by Type of End-Users
13.1.7.5. Pharmaceutical Contract Manufacturing Market in Middle East and North Africa, 2021-2030
13.1.7.5.1. Pharmaceutical Contract Manufacturing Market in Middle East and North Africa, 2021-2030: Distribution by Business Segment
13.1.7.5.2. Pharmaceutical Contract Manufacturing Market in Middle East and North Africa, 2021-2030: Distribution by Type of API Manufactured
13.1.7.5.3. Pharmaceutical Contract Manufacturing Market in Middle East and North Africa, 2021-2030: Distribution by Type of FDF Offered
13.1.7.5.4. Pharmaceutical Contract Manufacturing Market in Middle East and North Africa, 2021-2030: Distribution by Scale of Operation
13.1.7.5.5. Pharmaceutical Contract Manufacturing Market in Middle East and North Africa, 2021-2030: Distribution by Type of Packaging Form
13.1.7.5.6. Pharmaceutical Contract Manufacturing Market in Middle East and North Africa, 2021-2030: Distribution by Type of End-Users
- KEY INSIGHTS
14.1. Chapter Overview
14.2. Integrated Contract Service Providers (Heat Map Analysis)
14.3. Analysis by Geography
14.4. Analysis by Geography, Company Size and Business Segment
- SWOT ANALYSIS
15.1. Chapter Overview
15.2. Strengths
15.3. Weaknesses
15.4. Opportunities
15.5. Threats
15.6. Comparison of SWOT Factors
- CASE STUDY: COMPARISON OF SMALL AND LARGE MOLECULES DRUGS / THERAPIES
16.1. Chapter Overview
16.2. Small Molecule and Large Molecule Drugs / Therapies
16.2.1. Comparison of General Characteristics
16.2.2. Comparison of Key Specifications
16.2.3. Comparison of Manufacturing Processes
16.2.4. Comparison of Key Manufacturing-related Challenges
- CONCLUDING REMARKS
- EXECUTIVE INSIGHTS
18.1. Chapter Overview
18.2. Ajinomoto Althea
18.2.1. Company Snapshot
18.2.2. Interview Transcript: Scott Goldstein, Ex - Director, Business Development, Drug Product Manufacturing
18.3. Bachem
18.3.1. Company Snapshot
18.3.2. Interview Transcript: Thomas Fruh, Ex - Chief Executive Officer
18.4. CiVentiChem
18.4.1. Company Snapshot
18.4.2. Interview Transcript: Bhaskar VenePalli, President and Chief Executive Officer
18.5. CordenPharma
18.5.1. Company Snapshot
18.5.2. Interview Transcript: Roberto Margartia, Director, Business Development
18.6. Helsinn Group
18.6.1. Company Snapshot
18.6.2. Interview Transcript: Allison Vavala, Director, Commercial Development
18.7. Novasep
18.7.1. Company Snapshot
18.7.2. Interview Transcript: Kevin Daley, Director, Pharmaceuticals Marketing
18.8. Sovereign Pharma
18.8.1. Company Snapshot
18.8.2. Interview Transcript: Piyush Desai, Director, Operations
18.9. Wavelength Pharmaceuticals
18.9.1. Company Snapshot
18.9.2. Interview Transcript: Ilan Avni, Vice President Business Development, Marketing, and IP
- APPENDIX I: TABULATED DATA
- APPENDIX II: LIST OF COMPANIES AND ORGANIZATIONS
Contact Details
Roots Analysis Private Limited
Ben Johnson
+1 (415) 800 3415
+44 (122) 391 1091