Roots Analysis has done a detailed study on Cell Therapy Consumables Market, 2021-2031, covering key aspects of the industry’s evolution and identifying future growth opportunities.
To order this 230+ page report, which features 110+ figures and 120+ tables, please visit this –
https://www.rootsanalysis.com/reports/cell-therapy-consumables-market/request-sample.html
Key Market Insights
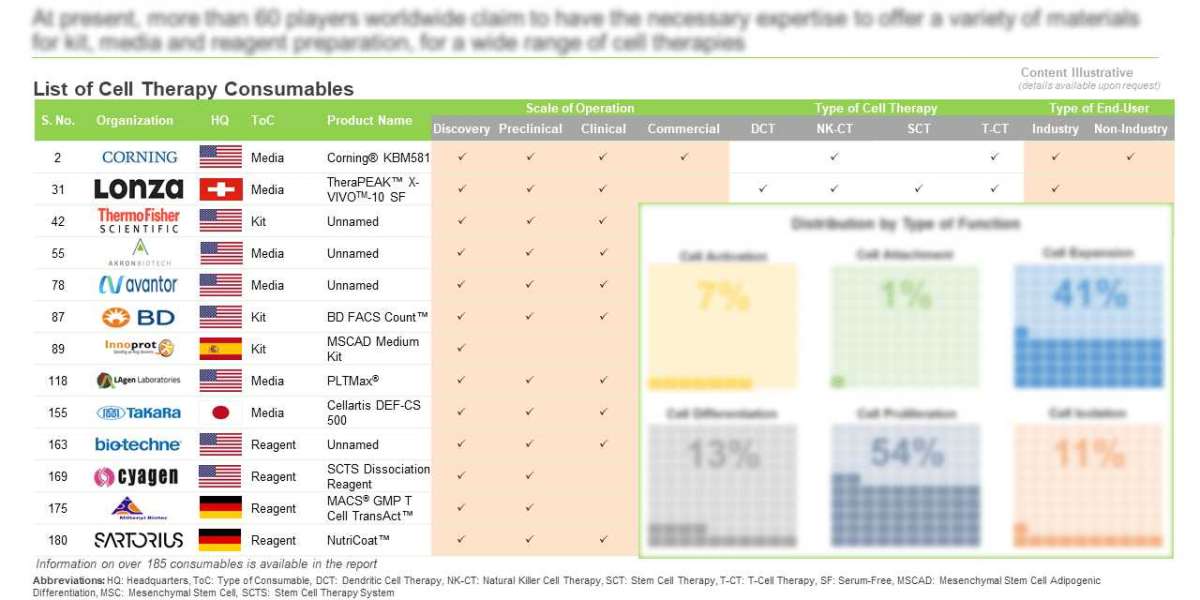
- At present, more than 60 players worldwide claim to have the necessary expertise to offer a variety of materials for kit, media and reagent preparation, for a wide range of cell therapies
- The market landscape is geographically well distributed, with several players offering consumable products for research, as well as therapeutic purposes
- Over time, a number of stakeholders have established strong brand positions; in future, such companies are anticipated to contribute the most to the overall revenues generation potential
- In pursuit of gaining a competitive edge, industry stakeholders are actively upgrading existing capabilities and enhancing their consumables-focused product portfolios
- To keep pace with the growing demand, many consumable providers have undertaken strategic initiatives, such as entering into mutually beneficial partnerships and expanding their capacities
- We expect industry stakeholders to continue to forge strategic alliances with niche / specialized players engaged in this domain to further augment their respective product offerings
- Presently, the demand for cell therapies exceeds the available capacity of developers; industry players will need to further increase the capacity to ensure consistent supply
- As the demand for cell therapies is indubitably rising, we anticipate the cell therapy consumables market to grow at an annualized rate of more than 17% in the near future
- The projected opportunity for cell therapy consumable providers is likely to be well distributed across different scales of operations, end-users and key geographical regions
For more information, please visit https://www.rootsanalysis.com/reports/cell-therapy-consumables-market.html
Table of Contents
- PREFACE
1.1. Scope of the Report
1.2. Research Methodology
1.3. Key Questions Answered
1.4. Chapter Outlines
- EXECUTIVE SUMMARY
2.1. Chapter Overview
- INTRODUCTION
3.1. Context and Background
3.2. Chapter Overview
3.3. Introduction to Cell Therapies
3.3.1. Comparison of Cell Therapies and Other Biotechnology Products
3.4. Classification of Cell Therapy Products
3.5. Overview of Cell Therapy Development and Manufacturing
3.6. Role of Raw Materials in Cell Therapy Development
3.7. Type of Cell Therapy Consumable
3.8. Key Challenges Associated with Cell Therapy Consumables
3.9. Future Perspectives
- MARKET LANDSCAPE
4.1. Chapter Overview
4.2. Cell Therapy Kit Providers: List of Players
4.2.1. Analysis by Year of Establishment
4.2.2. Analysis by Company Size
4.2.3. Analysis by Location of Headquarters
4.2.4. Analysis by Location of Kits Preparation Facilities
4.2.5. Analysis by Accreditations Received
4.2.6. Analysis by Type of End-User
4.2.7. Analysis by Type of Cell Therapy
4.2.8. Analysis by Type of Function
4.2.9. Analysis by Scale of Operation
4.2.10. Analysis by Application Area
4.2.11. Analysis by Application Area and Geography (Regional Landscape)
4.3. Cell Therapy Media Providers: List of Players
4.3.1. Analysis by Year of Establishment
4.3.2. Analysis by Company Size
4.3.3. Analysis by Location of Headquarters
4.3.4. Analysis by Location of Media Preparation Facilities
4.3.5. Analysis by Accreditations Received
4.3.6. Analysis by Type of End-User
4.3.7. Analysis by Type of Cell Therapy
4.3.8. Analysis by Media Components
4.3.9. Analysis by Type of Function
4.3.10. Analysis by Scale of Operation
4.3.11. Analysis by Application Area
4.3.12. Analysis by Application Area and Geography (Regional Landscape)
4.4. Cell Therapy Reagent Providers: List of Players
4.4.1. Analysis by Year of Establishment
4.4.2. Analysis by Company Size
4.4.3. Analysis by Location of Headquarters
4.4.4. Analysis by Location of Reagent Preparation Facilities
4.4.5. Analysis by Accreditations Received
4.4.6. Analysis by Type of End-User
4.4.7. Analysis by Type of Cell Therapy
4.4.8. Analysis by Type of Function
4.4.9. Analysis by Scale of Operation
4.4.10. Analysis by Application Area
4.4.11. Analysis by Application Area and Geography (Regional Landscape)
4.5. Analysis by Type of Consumable, Type of Cell Therapy and Application Area (Grid Representation)
- COMPANY COMPETITIVENESS ANALYSIS
5.1. Chapter Overview
5.2. Key Assumptions and Parameters
5.3. Methodology
5.4. Company Competitiveness: Kit Providers
5.5. Company Competitiveness: Media Providers
5.6. Company Competitiveness: Reagent Providers
- BRAND POSITIONING OF KEY INDUSTRY PLAYERS
6.1. Chapter Overview
6.2. Scope and Methodology
6.3. Bio-Techne
6.4. Miltenyi Biotec
6.5. Sartorius
6.6. STEMCELL Technologies
6.7. Thermo Fisher Scientific
- COMPANY PROFILES
7.1. Chapter Overview
7.2. Miltenyi Biotec
7.2.1. Company Overview
7.2.2. Product Portfolio
7.2.3. Recent Developments and Future Outlook
7.3. STEMCELL Technologies
7.3.1. Company Overview
7.3.2. Product Portfolio
7.3.3. Recent Developments and Future Outlook
7.4. Bio-Techne
7.4.1. Company Overview
7.4.2. Product Portfolio
7.4.3. Recent Developments and Future Outlook
7.5. Irvine Scientific
7.5.1. Company Overview
7.5.2. Product Portfolio
7.5.3. Recent Developments and Future Outlook
7.6. Thermo Fisher Scientific
7.6.1. Company Overview
7.6.2. Product Portfolio
7.6.3. Recent Developments and Future Outlook
7.7. Sartorius
7.7.1. Company Overview
7.7.2. Product Portfolio
7.7.3. Recent Developments and Future Outlook
7.8. BD Biosciences
7.8.1. Company Overview
7.8.2. Product Portfolio
7.8.3. Recent Developments and Future Outlook
7.9. Lonza
7.9.1. Company Overview
7.9.2. Product Portfolio
7.9.3. Recent Developments and Future Outlook
7.10. CellGenix
7.10.1. Company Overview
7.10.2. Product Portfolio
7.10.3. Recent Developments and Future Outlook
7.11. Corning
7.11.1. Company Overview
7.11.2. Product Portfolio
7.11.3. Recent Developments and Future Outlook
- RECENT DEVELOPMENTS AND INITIATIVES
8.1. Chapter Overview
8.2. Partnership Models
8.3. Cell Therapy Consumables: Recent Partnerships and Collaborations
8.3.1. Analysis by Year of Partnership
8.3.2. Analysis by Type of Partnership
8.3.3. Analysis by Year and Type of Partnership
8.3.4. Analysis by Type of Consumable
8.3.5. Analysis by Type of Partnership and Type of Consumable
8.3.6. Analysis by Type of Cell Therapy
8.3.7. Analysis by Type of Consumable and Type of Cell Therapy
8.3.8. Most Active Players: Analysis by Number of Partnerships
8.3.9. Analysis by Region
8.3.9.1. Intracontinental and Intercontinental Agreements
8.3.9.2. Local and International Agreements
8.3.10. Cumulative Year-wise Trend of Merger / Acquisition
8.3.11. Analysis by Type of Acquisition
8.3.12. Analysis by Key Value Drivers
8.3.13. Analysis by Year of Acquisition and Key Value Drivers
8.4. Cell Therapy Consumables: Recent Expansions
8.4.1. Analysis by Year of Expansion
8.4.2. Analysis by Purpose of Expansion
8.4.3. Analysis by Year and Purpose of Expansion
8.4.4. Analysis by Type of Consumable
8.4.5. Analysis by Purpose of Expansion and Type of Consumable
8.4.6. Analysis by Area of Expansion
8.4.7. Analysis by Region
8.4.7.1. Analysis by Location of Consumable Facility (Continent-wise)
8.4.7.2. Analysis by Location of Consumable Facility (Country-wise)
8.4.8. Analysis by Purpose of Expansion and Location of Headquarters
- LIKELY PARTNER ANALYSIS FOR CELL THERAPY CONSUMABLE PROVIDERS
9.1. Chapter Overview
9.2. Scoring Criteria and Key Assumptions
9.3. Scope and Methodology
9.4. Key Potential Strategic Partners for Cell Therapy Consumable Providers
9.3.1. Likely Partner Opportunities for Dendritic Cell Therapy Consumable Providers
9.3.2. Likely Partner Opportunities for NK Cell Therapy Consumable Providers
9.3.3. Likely Partner Opportunities for Stem Cell Therapy Consumable Providers
9.3.4. Likely Partner Opportunities for T-Cell Therapy Consumable Providers
- DEMAND ANALYSIS
10.1. Chapter Overview
10.2. Scope and Methodology
10.3. Global Demand for Cell Therapy Consumables
10.4. Global Demand for Cell Therapy Consumables for Planar Processes
10.5. Global Demand for Cell Therapy Consumables for Suspension Processes
10.6. Analysis by Scale of Operation
10.7. Analysis by Region
- MARKET FORECAST AND OPPORTUNITY ANALYSIS
11.1. Chapter Overview
11.2. Forecast Methodology
11.3. Global Outsourced Cell Therapy Consumables Market, 2021-2031
11.4. Outsourced Cell Therapy Consumables Market, 2021-2031: Distribution by Type of Consumable
11.4.1. Outsourced Cell Therapy Consumables Market for Kits, 2021-2031
11.4.2. Outsourced Cell Therapy Consumables Market for Media, 2021-2031
11.4.3. Outsourced Cell Therapy Consumables Market for Reagents, 2021-2031
11.5. Outsourced Cell Therapy Consumables Market, 2021-2031: Distribution by Type of Cell Therapy
11.5.1. Outsourced Cell Therapy Consumables Market for Dendritic Cell Therapy, 2021-2031
11.5.2. Outsourced Cell Therapy Consumables Market for NK Cell Therapy, 2021-2031
11.5.3. Outsourced Cell Therapy Consumables Market for Stem Cell Therapy, 2021-2031
11.5.4. Outsourced Cell Therapy Consumables Market for T-Cell Therapy, 2021-2031
11.6. Outsourced Cell Therapy Consumables Market, 2021-2031: Distribution by Scale of Operation
11.6.1. Outsourced Cell Therapy Consumables Market for Clinical Operations, 2021-2031
11.6.2. Outsourced Cell Therapy Consumables Market for Commercial Operations, 2021-2031
11.7. Outsourced Cell Therapy Consumables Market, 2021-2031: Distribution by Type of End-User
11.7.1. Outsourced Cell Therapy Consumables Market for Industry Players, 2021-2031
11.7.2. Outsourced Cell Therapy Consumables Market for Non-Industry Players, 2021-2031
11.8. Outsourced Cell Therapy Consumables Market, 2021-2031: Distribution by Geography
11.8.1. Outsourced Cell Therapy Consumables Market in North America, 2021-2031
11.8.2. Outsourced Cell Therapy Consumables Market in Europe, 2021-2031
11.8.3. Outsourced Cell Therapy Consumables Market in Asia-Pacific, 2021-2031
11.8.4. Outsourced Cell Therapy Consumables Market in Rest of the World, 2021-2031
- UPCOMING TRENDS AND FUTURE GROWTH OPPORTUNITIES
12.1. Chapter Overview
12.2. Emerging Trends Related to Cell Culture Media
12.3. Automation of Cell Therapy Manufacturing Processes
12.4. Single Use Systems and Technologies in Cell Therapy Manufacturing
- IMPACT OF COVID-19 ON CELL THERAPY CONSUMABLES MARKET
13.1. Chapter Overview
13.2. Impact of COVID-19 Pandemic on Cell Therapy Consumables Market
13.3. Impact on Future Market Opportunities for Cell Therapy Consumable Providers
13.4. Current Opinions and Key Initiatives of Key Players
13.5. Recuperative Strategies for Developer Businesses
13.5.1. Strategies for Implementation in the Short / Mid Term
13.5.2. Strategies for Implementation in the Long Term
- CONCLUDING REMARKS
14.1. Chapter Overview
- INTERVIEW TRANSCRIPTS
15.1. Chapter Overview
15.2. Anant Kamath, Chief Operating Officer, Cellular Engineering Technologies
15.2.1. Cellular Engineering Technologies: Key Highlights
15.2.2. Interview Transcript
15.3. Vishal G. Warke, Director RD, Cell Culture and Immunology, HiMedia Laboratories and Gauri W. Page, Assistant RD Manager, Animal Cell Culture, Himedia Laboratories
15.3.1. HiMedia Laboratories: Key Highlights
15.3.2. Interview Transcript
- APPENDIX I: TABULATED DATA
- APPENDIX II: LIST OF COMPANIES AND ORGANIZATIONS
Contact Details
Ben Johnson
+1 (415) 800 3415