Given the consistent increase in number of cell therapies being developed and launched, this upcoming therapeutic segment is on its way to becoming one of the highest valued markets within the biopharmaceutical industry
London
Roots Analysis has announced the addition of “Cell Therapy Manufacturing Market (4th Edition), 2021-2030” report to its list of offerings.
Owing to the complex manufacturing processes, requirement of advanced production facilities and the growing demand for cell therapy products, developers are actively outsourcing certain manufacturing operations, in addition to expanding their in-house capabilities.
To order this 620 page report, which features 210+ figures and 280+ tables, please visit https://www.rootsanalysis.com/reports/view_document/cell-therapy-manufacturing/285.html
Key Market Insights
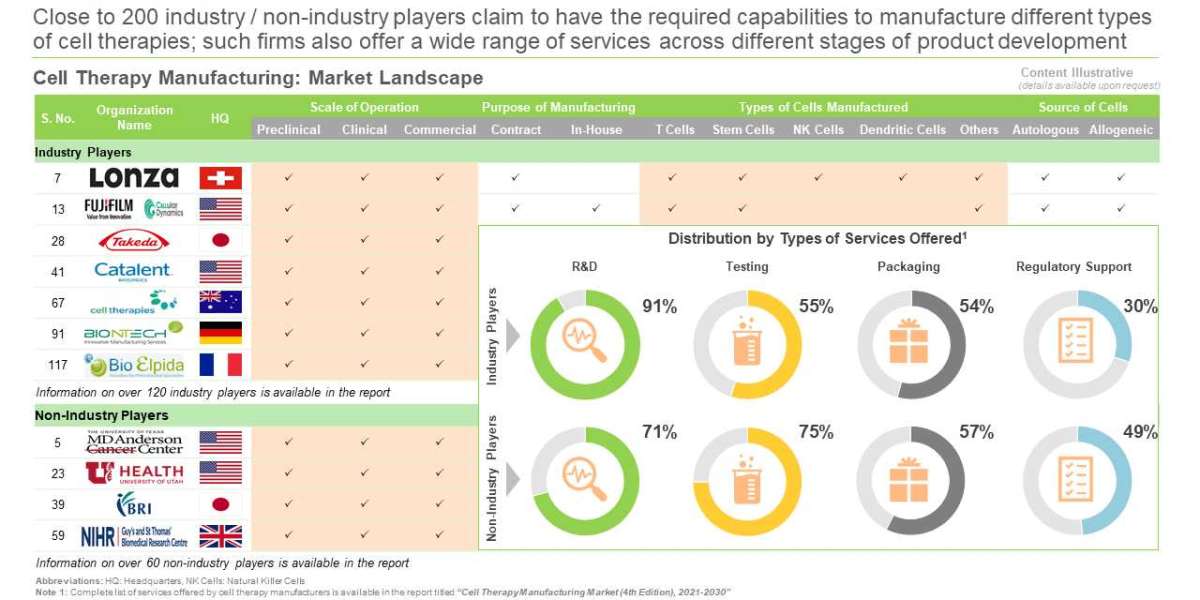
Around 200 organizations claim to be engaged in cell therapy manufacturing
The market landscape is dominated by industry players, which constitute 65% of the total number of stakeholders. Amongst these, over 25% companies are large firms.
280+ production facilities dedicated to cell therapies have been established worldwide
North America has emerged as the manufacturing hub for cell therapies, with the presence of nearly 45% of the manufacturing facilities; this is followed by Europe (31%). Other emerging regions include China, Japan, South Korea and Australia.
90 cell therapy manufacturers are focused on immune cell and stem cell therapies
Most of the players in this domain are focused on manufacturing of T cell therapies, primarily CAR-T therapies, while the stem cell therapy manufacturers are primarily engaged in the production of adult stem cells and mesenchymal stem cell therapies
Presently, more than 70 companies carry out manufacturing at all scales of operation.
Nearly 45% players have the required capabilities for commercial scale manufacturing. It is worth noting that all the industry players manufacture cell therapies required for clinical purposes.
35+ companies offer automated and closed systems to cell therapy developers
More than 60 automated and closed systems are being used for cell therapy manufacturing. Organizations that are presently offering customized automated solutions for cell therapy processes / manufacturing are Fraunhofer Institute for Manufacturing Engineering and Automation IPA (Germany), KMC Systems (US), RoosterBio (US) and Mayo Clinic Center for Regenerative Medicine (US).
Several partnerships were established in this domain, during the period 2016-2021
More than 180 deals have been inked during the given time period. A large proportion (34%) of the partnerships were related to manufacturing of cell therapies, followed by acquisitions (17%) and licensing agreements (14%).
Expansion activity in this domain has grown at a CAGR of 59%, between 2016 and 2021
More than 75 facility expansions were reported during the given time period. Over 80% instances were related to the establishment of new facilities, followed by those involving the expansion of existing facilities (17%).
Role of big pharma players in this industry has evolved over the last few years; their initiatives increased at a CAGR of 41% during the period 2016-2020
Several big pharma players have undertaken various initiatives focused on cell therapy manufacturing. Gilead sciences, Takeda Pharmaceutical and Novartis are some of the prominent big pharma players in this domain.
The currently available global cell therapy manufacturing capacity is estimated to be over 1.88 billion sq. ft. of dedicated cleanroom area
The maximum (48%) installed capacity (in terms of cleanroom area) belongs to companies based in North America (48%); the region has higher number of players having multiple production facilities. This is followed by Asia Pacific (29%) and Europe (23%).
The demand for cell therapies is anticipated to grow at a CAGR of 22%, during 2021-2030
Presently, the clinical demand for stem cell and CAR-T cell-based products is the highest; this trend is unlikely to change in the foreseen future as well. On the other hand, the demand for tumor cell, NK cell and dendritic cell therapies is expected to grow at a relatively faster pace, over the next decade.
By 2030, the market for commercial scale cell therapy manufacturing is likely to grow at an annualized rate of 31.5%
Currently, North America and Europe capture more than 70% share of the overall market. Specifically, the cell therapy manufacturing market in Asia Pacific is driven by countries, such as China, Japan, South Korea, India and Singapore. It is worth noting that the current market in Asia Pacific is primarily driven by the clinical demand for cell therapies.
To request a sample copy / brochure of this report, please visit https://www.rootsanalysis.com/reports/view_document/cell-therapy-manufacturing/285.html
The USD 14.5 billion (by 2030) financial opportunity associated with cell therapy manufacturing market has been analyzed across the following segments:
- Type of Cell Therapy
- T cell therapies
- Dendritic and tumor cell therapies
- NK cell therapies
- Stem cell therapies
- Other ATMPs
- Source of Cell
- Autologous
- Allogeneic
- Scale of Operation
- Clinical
- Commercial
- Purpose of Manufacturing
- In-house Manufacturing
- Contract Manufacturing
- Geographical Regions
- North America
- Europe
- Asia Pacific
- Rest of the World
The report also features inputs from eminent industry stakeholders, according to whom, the manufacturing of cell therapies is largely being outsourced due to exorbitant costs associated with the setting-up of in-house expertise. The report includes detailed transcripts of discussions held with the following experts:
- Troels Jordansen (Chief Executive Officer, Glycostem Therapeutics)
- Gilles Devillers (General Manager, Bio Elpida)
- Wei (William) Cao (Chief Executive Officer, Gracell Biotechnologies)
- Arik Hasson (Executive VP Research and Development, Kadimastem)
- Fiona Bellot (Business Development Manager, Roslin CT)
- David Mckenna (Professor and American Red Cross Chair in Transfusion Medicine, University of Minnesota)
- Victor Lietao Li (Co-Founder and Chief Executive Officer, Lion TCR)
- Arnaud Deladeriere (Manager, Business Development Operations-cGMP Manufacturing Unit, C3i Center for Commercialization of Cancer Immunotherapy)
- Brian Dattilo (Manager of Business Development, Waisman Biomanufacturing)
- Mathilde Girard (Department Leader, Cell Therapy Innovation and Development, Yposkesi)
- Tim Oldham (Chief Executive Officer, Cell Therapies)
- Gerard MJ Bos (Chief Executive Officer, CiMaas)
For additional details, please visit
https://www.rootsanalysis.com/reports/view_document/cell-therapy-manufacturing/285.html or email sales@rootsanalysis.com
You may also be interested in the following titles:
- Biologics Fill / Finish Services Market (2nd Edition): Industry Trends and Global Forecasts, 2021-2030
- Vaccine Contract Manufacturing Market (3rd Edition): Industry Trends and Global Forecasts, 2021-2030
- TIL-based Therapies Market: Industry Trends and Global Forecasts, 2021-2030
- TCR-based Therapies Market: Industry Trends and Global Forecasts, 2021-2030
- Global T-Cell (CAR-T, TCR, and TIL) Therapies Market (5th Edition): Industry Trends and Global Forecasts, 2021-2030
Contact:
Gaurav Chaudhary
+1 (415) 800 3415
+44 (122) 391 1091
Gaurav.Chaudhary@rootsanalysis.com
Roots Analysis
Web: https://www.rootsanalysis.com/
LinkedIn: https://in.linkedin.com/company/roots-analysis
Twitter: https://twitter.com/RootsAnalysis.com
Medium: https://medium.com/@RootsAnalysis
Pinterest: https://in.pinterest.com/RootsanalysisPin/_saved/