Roots Analysis has announced the addition of Pre-Sterilized / Ready-to-Use Primary Packaging Market report to its list of offerings.
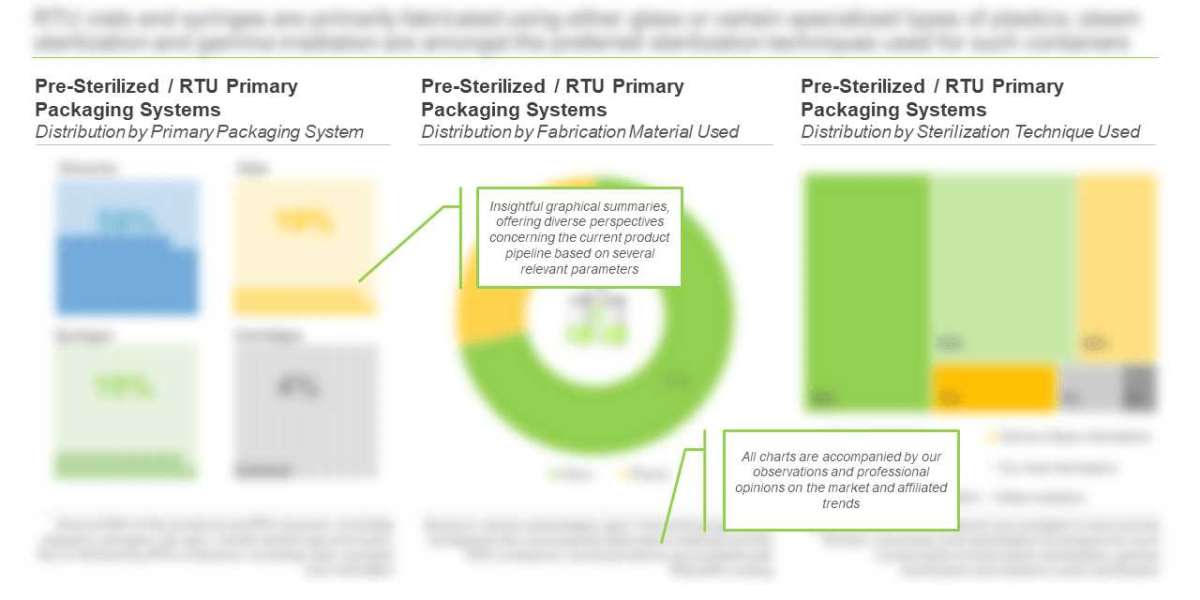
Presently, more than 150 pre-sterilized / RTU primary packaging systems, including cartridges, syringes and vials, as well as their respective closure systems are available worldwide. Majority (50%) of the pre-sterilized / RTU primary packaging systems are closures, followed by containers (34%) and container-closure systems (16%). It is worth highlighting that, of the total, 54 closures are suitable for use with vials, 19 are suitable for use with syringes and the remaining 5 are compatible with cartridges.
To request a sample copy / brochure of this report, please visit this – https://www.rootsanalysis.com/reports/224/request-sample.html
Amongst the primary packaging containers, vials and syringes emerged as the most common types of pre-sterilized / RTU containers manufactured by stakeholders engaged in this domain. This can be attributed to the fact that these containers are the most suitable for packaging of small volume of therapeutics, such as biologics, cell therapies, small molecules, vaccines and other potent products.
Scope of the Report: -
The “Pre-Sterilized / Ready-to-Use Primary Packaging: Focus on Cartridges, Syringes and Vials (2nd Edition) - Market Distribution by Type of Packaging System (Vials, Syringes, Cartridges and Closures), Packaging Material (Glass and Plastic) and Key Geography (North America, Europe, Asia Pacific, Latin America, Middle East and North Africa and Rest of the World) – Global Forecast 2021-2030” report features an extensive study of the current market landscape and future potential of ready-to-use primary packaging components, including cartridges, syringes and vials, and their respective closure systems (such as stoppers, needle shields and plungers) over the next decade. The study features an in-depth analysis of the key drivers and trends related to this domain. Amongst other elements, the report includes:
- A detailed assessment of the current market landscape of ready-to-use primary packaging components, featuring information on the type of container (vials, syringes, cartridges and others), type of fabrication material used (glass and plastic), sterilization technique used (ethylene oxide sterilization, steam sterilization, gamma irradiation, electron beam sterilization and dry heat sterilization), volume of container (less than 50 ml, 50 ml to 100 ml and more than 100 ml), compatible drug class (biologics and small molecules) and type of closure (stoppers, plungers, tip caps / needle shields, caps and seals). In addition, the chapter includes details related to ready-to-use packaging system manufacturers, along with information on their respective year of establishment, company size, location of headquarters and key players (in terms of number of offerings).
- A competitiveness analysis of ready-to-use primary packaging system manufacturers based on various relevant parameters, such as supplier power (based on the experience / expertise of the manufacturer) and key product specifications (type of container, type of fabrication material used, type of closure, sterilization technique used and compatible drug class).
- Elaborate profiles of prominent players engaged in the development of ready-to-use primary packaging systems. Each profile features a brief overview of the company, details related to its financials (if available), product portfolio, recent developments and an informed future outlook.
- An analysis of the partnerships that have been inked by stakeholders engaged in this domain, during the period 2015-2021, covering acquisitions, product / technology integration agreements, supply agreements, product / technology licensing agreements, product / technology development agreements, service alliances, distribution agreements and other relevant types of deals.
- An insightful discussion on the impact of the recent COVID-19 pandemic on the businesses of primary pharmaceutical packaging manufacturers. In addition, we have provided information on the strategic initiatives undertaken by pharmaceutical players to mitigate the challenges affiliated with the current global crisis.
- An in-depth analysis to estimate the current and future demand for ready-to-use container-closure systems based on various relevant parameters, such as type of packaging system and type of fabrication material used, across different regions, for the period 2021-2030.
- An elaborate discussion on the key trends (such as the advent of personalized / targeted therapies, shift towards more flexible packaging, rise of modular manufacturing facilities and advancement in robotic packaging solutions) that are likely to have an impact on the future adoption of ready-to-use packaging systems. It also features a Harvey ball analysis, highlighting the relative effect of each trend on the overall pharmaceutical packaging industry.
- A case study on the use of robotic machinery in pharmaceutical manufacturing and fill / finish operations, highlighting the advantages of using automation / automated technologies in such processes. Further, it presents the profiles of industry players that provide such equipment for aseptic processing of pharmaceuticals.
Key Questions Answered
- Who are the prominent players engaged in the global pre-sterilized / ready-to-use primary packaging market?
- What is the relative competitiveness of different ready-to-use primary packaging system manufacturers?
- Which partnership models are most commonly adopted by stakeholders engaged in this industry?
- How is the recent COVID-19 pandemic likely to impact the global ready-to-use primary packaging systems market?
- What is the current, global demand for ready-to-use containers and closures?
- What are the major market trends and driving factors that are likely to impact the growth of ready-to-use primary packaging systems market?
- How is the current and future opportunity likely to be distributed across key market segments?
To request a free insight to this report – https://www.rootsanalysis.com/reports/224/free-insights.html
Sterilization of primary packaging systems is considered to be an essential step in the overall healthcare manufacturing process as it deactivates various forms of contaminating agents (particularly referring to bacteria, fungi, protozoa and viruses). Majority (43%) of the pre-sterilized / RTU containers are sterilized using ethylene oxide, followed by those employing steam sterilization (34%).
For additional details, please visit - https://www.rootsanalysis.com/reports/view_document/pre-sterilized-ready-to-use-primary-packaging-market/224.html Or sales@rootsanalysis.com
Contact Details
Ben Johnson
+1 (415) 800 3415
ben.johnson@rootsanalysis.com
Roots Analysis
Web: https://www.rootsanalysis.com/
LinkedIn: https://in.linkedin.com/company/roots-analysis
Twitter: https://twitter.com/RootsAnalysis.com
Medium: https://medium.com/@RootsAnalysis
Pinterest: https://in.pinterest.com/RootsanalysisPin/_saved/